No new therapy without a clinical trial
Clinical trials
At present, no ground-breaking therapies exist for any form of EB. Although the current number of EB clinical trials being undertaken is still relatively small, the diversity of therapeutic approaches in preclinical development and entering clinical trials is growing rapidly.
In 2006, there was one pilot clinical trial for an ex vivo gene therapy in one patient – the first time there was proof that the underlying genetic fault in one type of EB could be corrected. Today, many different approaches to therapy are underway or planned, for all types of EB, including cell-, gene-, protein- RNA-based, and drug therapies.
2022 will go down as a milestone in the history of EB research. The first drug approved for EB was approved in the EU (Filsuvez®). The largest clinical trial worldwide involved over 50 clinical centers and more than 20 countries. The positive approval shows that the development of EB-specific drugs and therapies is possible and provides tailwind for further success.
The two main types of clinical studies in EB research are interventional and observational. Both are important to move research forward and provide the best outcomes for people with EB.
Interventional studies
Commonly referred to as clinical trials, interventional studies are prospective studies tailored to evaluate the direct impact of treatment or preventative measures in EB patients. This can involve a potential drug, medical device, activity, or procedure.
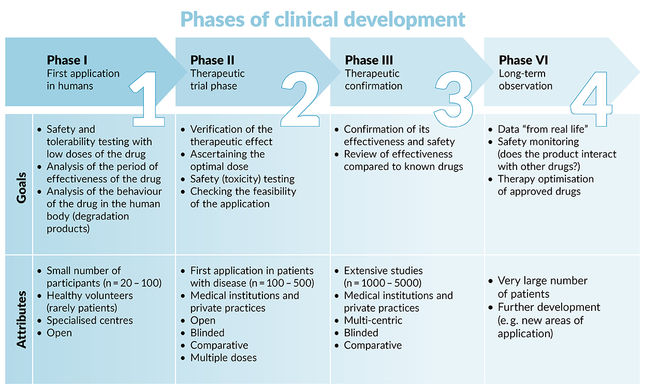
New potential drugs need to pass through three phases (1, 2, 3) of interventional testing to show that they are safe and effective before receiving approval from the U.S. Food and Drug Administration (FDA) or European Medicines Agency (EMA). After a drug is approved, further studies (phase 4) will be performed to monitor for safety and effectiveness.
Observational studies
Besides testing new therapy approaches for EB patients in clinical trials, observational studies, in which researchers observe participants and track health outcomes over time, are an important category of study designs, to address investigative questions. Well-designed observational cohort studies in EB are helpful in evaluating clinical and epidemiologic characteristics, phenotype-genotype correlations, natural history of the disease, molecular or cellular signatures, impact of treatment strategies and secondary outcomes of clinical studies. Further, basic science studies utilize hypothesis-driven experimental designs to improve the knowledge and understanding of underlying disease mechanisms in EB.
Database for EB clinical trials
EB Resnet members prepared a database summarising all interventional and observational studies involving EB, that are registered on Clinicaltrials.gov, the EU Clinical Trials Register, the International Clinical Trials Registry Platform (ICTRP), and the UMIN Clinical Trials Registry.
DEBRA International
DEBRA International is the umbrella organisation for more than 50 national DEBRA groups. Find out more
EB Clinet
EB Clinet – Clinical Network for Epidermolysis Bullosa, is a network of EB centres and experts which aims to improve medical care for people with EB and to accelerate the way to a cure by connecting health care professionals working with EB for the purpose of sharing of knowledge and experience. Find out more